1. Define all words
2a. P- phase change
2b. C- oxidation
2c. P- Phase change
2d. C- chem. reaction
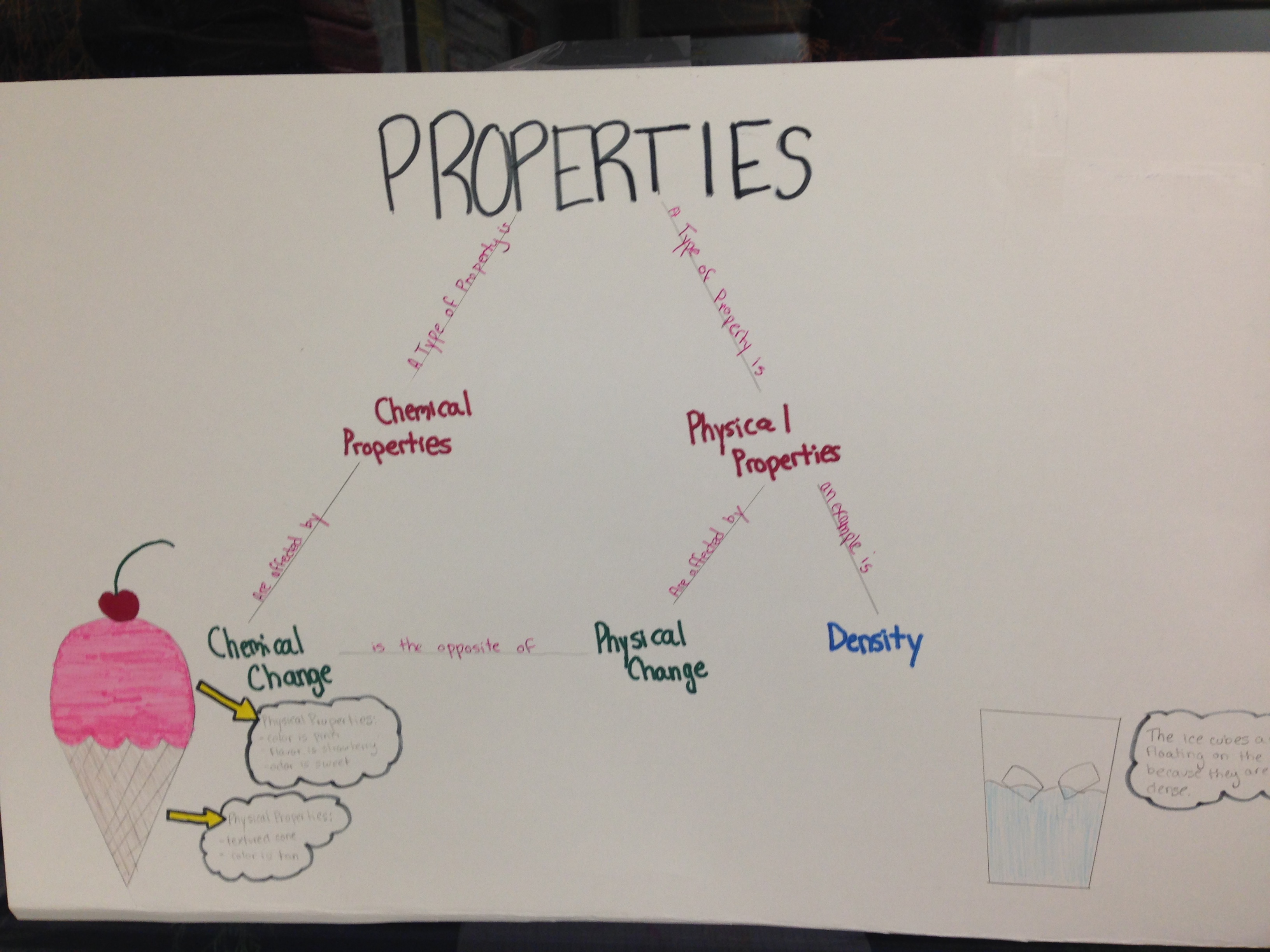
3. Melting point, density, color, state: physical changes
4. carbon (PS, E); water (PS, C); marble (M, HT, Sus.); diamond (PS, E); ocean water (M, HT, Sus.); #2 pencil (M, HT); chocolate chip ice cream (M, HT, Sus.); gold (PS, E); hand cream (M, HM, Sol’n); NaCl+H2O (M, HM, Sol’n); PowerAde (M, HM, Sol’n); Frappuccino (M, HM, Sol’n); Brass (M, HM, Sol’n); NaCO3 (PS, C); Vinagrette (M, HT, Sus.)
5. See pg 82-83, section 3.3: Filtration (to separate solids from liquids); Distillation (to separate two liquids with varying boiling point); Crystallization (to separate solute from solvent in a solution); Sublimation (to separate a mixture of solids when one of the solids can sublime); chromatography (to separate two substances in a solution)
6a. 70g
6b. the “3” grams was not lost, it was converted into a gas, which was not trapped and measured
6c. If the gas given off from the candle was trapped and its mass was measured, it would have a mass of 3g. Therefore, the mass before the reaction (mass of total reactants, 73g) is equal to the mass after the reaction (mass of total products 73g)
7. C= 42.86%, O= 57.86%
8. %O= 72%. The two compounds are not the same because they don’t contain the %Oxygen by mass, in accordance with the Law of Definite Proportions. (57% O by mass for cmpd 1; 72% O by mass for cmpd 2)
9. mass ratio for cmpd 1: 0.75gofC/gofO; mass ratio for cmpd 2: 0.37gofC/gofO; The two compounds are different. The whole number ratio of cmpd1:cmpd2 in accordance with Law of Multiple Proportions is 2: cmpd 1 has 2x more gofC/gofO than cmpd 2 (0.75gofC/GofO per 0.37gofC/GofO= 2)
Do you need more practice problems? Can you just not get enough!!??!! See problems 71, 73-80, and 93 at the end of chapter 3. Answers posted below!!! Your welcome 🙂
71. LoDP
73. NaCl= 1:1, CuO= 1:1, H2O= 2:1, H2O2= 1:1
74. 3.16% –> 3% w. SF
75. 39.7% (3SF)
76. 92.96% –> 93% w. 3SF
77. cmpd1: 0.75gC/gO; cmpd2: 0.37gC/gO; ratio of cmpd1:cmpd2: whole number = 2
78. 64%
79. Law of Multiple Proportions. CO2= higher percent by mass of O because more O atoms
80. CuO: 19%, 68.0g; H2O: 89%, 1.98g; H2O2: 94%, 32.0g; CO: 57%, 12.0g; CO2: 73%, 11.9g
93a. Samples I, IV, and III are from the same compound bc they have the same mass ratio of y:x (as shown by the same slope)
93b. approximate ratio: ~0.25gY/gX
93c. approximate ratio: ~0.5gY/gX